12 May Teratogenicity Testing: Harnessing the Power of Zebrafish for Efficient and Accurate Drug Screening
Teratogenicity testing is a crucial step in drug development to identify substances that can cause physical or functional defects in human embryos or fetuses. Following the Thalidomide tragedy in the 1950s and 1960s, it became mandatory to assess a drug’s potential to induce congenital defects through teratogenic assays. With the increasing development of chemicals for various purposes, such as treatment, cosmetics, and food additives, there has been a significant rise in the number of compounds requiring teratogenicity testing.
Conventional animal tests for assessing developmental and reproductive toxicity are time-consuming, costly, and labor-intensive. Therefore, there is a growing need to develop alternative teratogenic assays that are cost-effective, offer high throughput, are easy to conduct, provide reproducible results, and align with in vivo mammalian data.
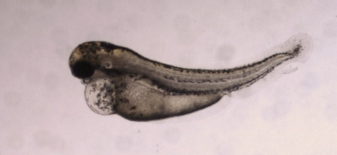
The zebrafish model emerges as an attractive candidate for early drug development screening of developmental toxicity. Zebrafish are cost-effective and easy to maintain, allowing for High Content Screening (HCS) of drug candidates. Additionally, zebrafish development closely resembles that of mammals, and many molecular pathways are conserved between zebrafish and humans, making them highly predictive of mammalian responses. One notable example of a High-Content teratogenicity assay is the Teratotox Assay offered by Biobide.
Teratogens are substances that can cause physical or functional defects in human embryos or fetuses when pregnant women are exposed to them. These substances can affect the embryo or fetus in various ways, leading to physical malformations, developmental issues in behavior or emotions, and defects in the nervous system that may result in disability or retardation. Teratogens can also cause complications during pregnancy, such as preterm labor, spontaneous abortions, or miscarriages.
The thalidomide controversy of the 1950s is a well-known example of developmental toxicity. Thalidomide, initially marketed in 46 countries to treat morning sickness, colds, and flu in pregnant women, led to numerous birth defects. The absence of tests on pregnant women before its release falsely assured its safety until deformed children were born. This incident prompted regulatory changes, and the Food and Drug Administration (FDA) issued guidelines for evaluating teratogenicity, categorizing drugs into classes A, B, C, D, and X based on their associated risks during pregnancy. Drugs falling into categories D and X have embryotoxic and/or teratogenic effects on humans and animals. The European Medicines Agency (EMEA) has a similar classification system, described in the ICH S5(R3) Guideline, for assessing reproductive toxicity in human pharmaceuticals during drug discovery and development or before commercialization.
Traditional teratogenicity testing involves extensive animal testing using pregnant laboratory animals, including mice, rats, rabbits, and non-human primates. Test compounds are administered daily during the period of fetal organogenesis, and the fetus is examined for skeletal, visceral, and external anomalies near term. However, these tests are costly, time-consuming, and contradict the demand for rapid testing. Additionally, there is increasing pressure from the public and regulatory bodies to reduce animal use, leading to the need for alternative test systems.
In vitro models using cells and tissues have been developed as alternatives to animal testing, following the 3Rs principle of Replacement, Reduction, and Refinement. These include whole embryo culture tests, organ culture teratogen assays, and eukaryotic cell culture systems. However, in vitro assays often fail to predict in vivo results involving drug absorption, distribution, metabolism, and excretion (ADME).
Zebrafish have gained popularity as an alternative animal model for in vivo drug and chemical toxicity assays. They offer significant advantages over conventional mammal testing. Zebrafish embryos are small in size, highly fertile, and undergo rapid development and organogenesis, with most organs developed within 120 hours post-fertilization. This allows for shorter testing periods, lower costs, smaller compound quantities, easy manipulation, direct compound delivery, and scalability for High Content Screening. Moreover, zebrafish experiments can be conducted during embryonic development under the 3Rs principles without the need for approval from Ethical Committees. Their transparency at the larval stage also enables non-invasive procedures.
Furthermore, zebrafish share similarities in physiology, development, metabolism, and pathways with mammals. They possess orthologues to a large majority (86%) of human drug targets and share approximately 85% homology with humans in terms of their genome. Numerous studies have demonstrated the high predictability of zebrafish responses to toxic substances for mammalian responses.
Recognizing the potential of zebrafish as an experimental organism, the National Institutes of Health (NIH) ranked them as the third most important organism after rats and mice in 2003. Regulatory agencies such as the FDA and EMEA have also accepted zebrafish toxicity and safety assessment data for drug approval, offering an alternative to the conventional screening models using rodents and rabbits.
Compared to other vertebrate models, zebrafish complete embryogenesis within the first 72 hours and develop discrete organs and tissues by 120 hours post-fertilization. This aligns with the critical organ formation period in rats. Quantitative prediction of the teratogenic potential of compounds in zebrafish embryos can be achieved through a scoring system based on phenotypic changes, similar to mammalian in vivo embryo-fetal development assessments. The zebrafish model serves as an alternative developmental toxicology model for predicting effects in humans and can be complemented by sperm analysis to obtain information on both embryo development and spermatogenesis.
Biobide’s Teratotox Assay in zebrafish is a valuable tool for evaluating the teratogenic properties of potential drugs during the early preclinical phase. This assay offers a cost-effective and High Content format, producing highly transferable results to other vertebrates and humans. Zebrafish embryos expressing a green fluorescent protein in the heart are used in strict environmental conditions. The embryos are treated with test compounds, and various organs and processes are analyzed to identify malformations, morphological defects, edema, necrotic tissue, and other teratogenic and toxic endpoints. Parameters such as EC50, LC50, Teratogenic Index (TI), and NOAEL values are calculated.
Additional analysis techniques, including bioavailability assessment through HPLC-MS/MS and histopathology, can be employed to further investigate the internal concentration of test compounds and ensure accurate results. By utilizing zebrafish as an intermediate step between cell-based evaluation and mammalian animal testing, the Teratotox Assay enhances the speed, cost-efficiency, and accuracy of teratogenicity assessment in drug development.
In conclusion, teratogenicity testing is a crucial aspect of drug development to ensure the safety of potential drugs for pregnant women and their developing fetuses. The zebrafish model offers a promising alternative to traditional mammal testing, providing cost-effective, high-throughput, and predictive assessments of developmental toxicity. The use of zebrafish in assays such as Biobide’s Teratotox Assay presents a valuable tool for screening drugs for birth defects and facilitating safer pharmaceutical development.



No Comments